Pharmaceutical
From Sample Preparation to Analysis, We Have You Covered
The analysis of pharmaceutical molecules provides challenges which we aim to help you overcome. From sample preparation of PK/ADME samples, to complex HPLC/UHPLC separations and residual solvent analysis we have the solutions you need. We provide:
- Method development/pharma workflow support,
- Troubleshooting for sample preparation, HPLC and GC
- Cost effective analytical solutions.
Discover a variety of pharmaceutical solutions tailored to you. You can also easily access the Nitrosamines and NDSRI application portal.

Therapeutic Peptides
Therapeutic peptides are short chains of amino acids that have emerged as a promising class of drugs due to their high specificity, potency, and favorable safety profiles. They mimic natural peptides in the body, allowing them to engage with specific receptors and pathways involved in various physiological processes. This makes them particularly effective in treating a wide range of conditions, including metabolic disorders, cancer, and infectious diseases. Their small size and ability to be easily modified for improved stability and delivery further enhance their therapeutic potential. As a result, therapeutic peptides are becoming increasingly important in the development of novel treatments with fewer side effects compared to traditional small molecule drugs.
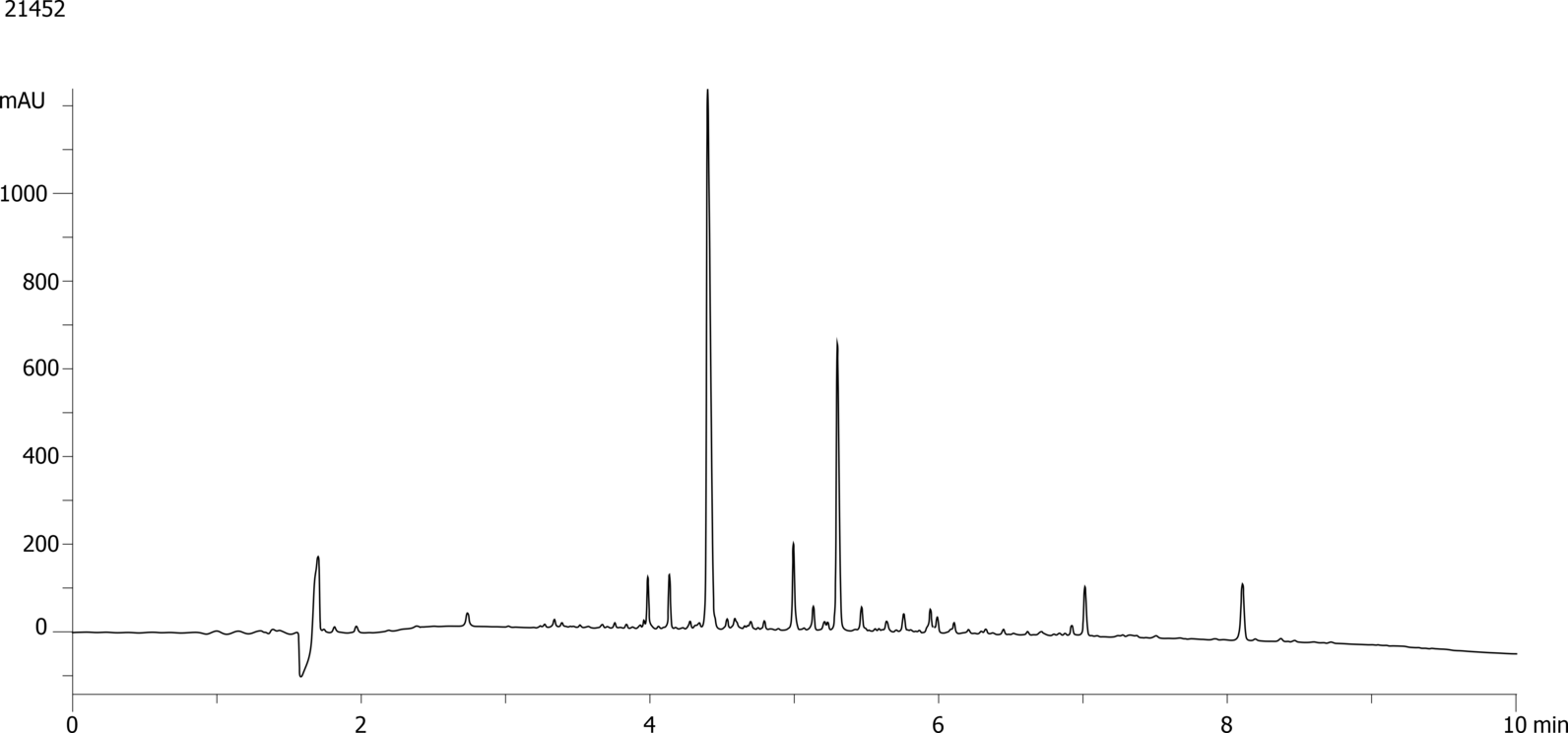
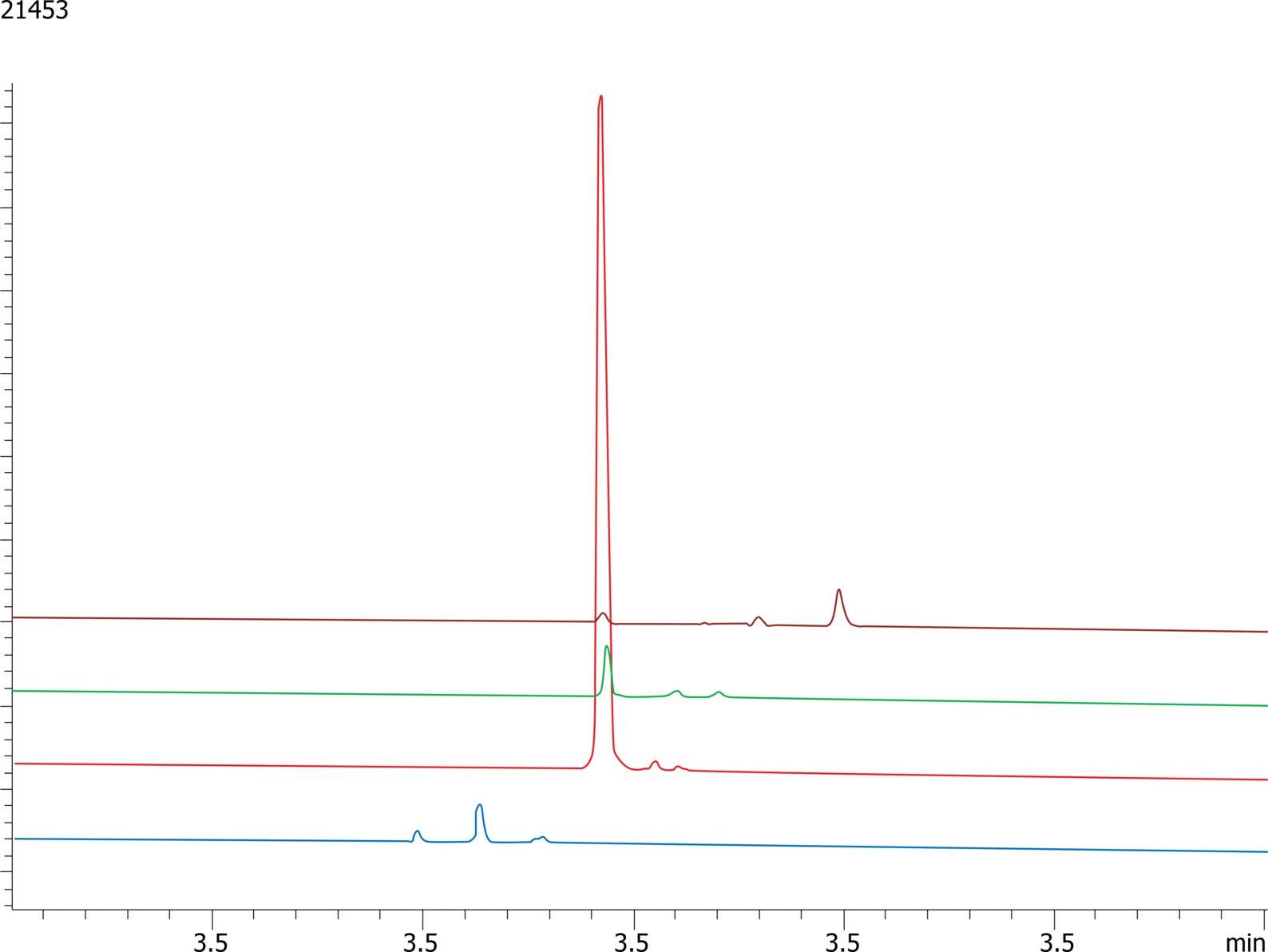
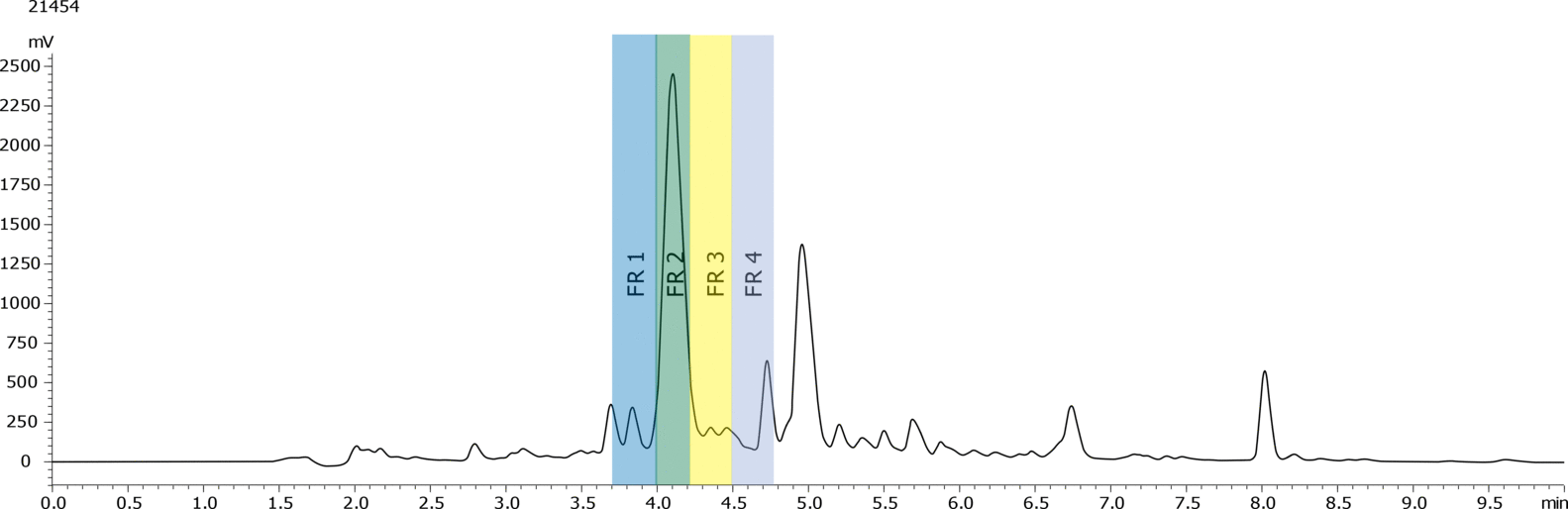
High-Performance Liquid Chromatography (HPLC) is a vital technique for purifying therapeutic peptides, ensuring their efficacy and safety. By exploiting differences in peptide properties such as size, charge, and hydrophobicity, HPLC effectively separates and isolates the desired peptide from impurities and by-products. The method's high resolution and precision make it ideal for producing peptides with high purity, which is crucial for clinical applications. Additionally, HPLC can be scaled up for industrial production, providing a reliable and consistent means of purification. This ensures that therapeutic peptides meet stringent regulatory standards, ultimately leading to more effective and safer treatments for patients.
GLP-1 (Glucagon-Like Peptide-1) analogues are a specific focus, with their potential to regulate blood sugar levels, slowing gastric emptying and promoting a feeling of fullness. Several drugs are already on the market (semaglutide, dulaglutide, exenatide and liraglutide) and finding successful strategies for the purification and quality assurance of these therapeutic peptides is of current interest to many pharmaceutical companies.
Trending Resources
Applications