Reversed Phase HPLC
Reversed-phase Liquid Chromatography (RP-LC)
Being the most common principle of HPLC/UHPLC separation mode, reversed-phase liquid chromatography offers retention of compounds with hydrophobic and organic functionality. Retention of these compounds by reversed-phase liquid chromatography involves a combination of hydrophobic and van der Waals-type interactions between each target compound and both the stationary phase and mobile phase.
Stationary phases used in reversed-phase chromatography typically consist of varying lengths of hydrocarbons such as C18, C8, and C4 or strongly hydrophobic polymers such as styrene divinylbenzene.
- C18 HPLC columns are the most preferred as they offer an excellent range of hydrophobic separation power along with high surface area coverage.
- C8 HPLC column are used when less retention compared to a C18 is needed.
- C4 and C5 HPLC columns are commonly used to separate large macromolecules such as proteins.
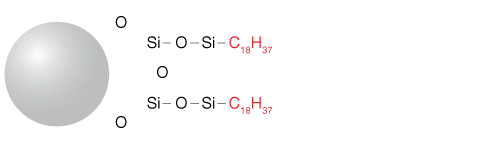
Phenomenex’s Product Offering in Reversed-phase Columns
Looking for high-performance reversed-phase columns tailored to your application? Phenomenex offers a leading portfolio of reversed-phase HPLC and UHPLC columns, engineered for exceptional resolution, reproducibility, and speed.
Explore our full range of reversed-phase columns with a wide range of large and small molecule reversed-phase brands and find the best match for your analytes and system needs.
How Reversed-phase Liquid Chromatography Works?
Reversed-phase liquid chromatography is a dominant analytical technique that separates compounds based on their hydrophobicity using a non-polar stationary phase and a polar mobile phase. It is the most widely used mode of high-performance liquid chromatography (HPLC), especially in biomedical and pharmaceutical applications.
Stationary Phase
The stationary phase typically consists of hydrophobic materials chemically bonded to a variety of base materials, including fully porous silica, core-shell silica, polymeric particles, and hybrid organosilica materials. Common bonded hydrocarbons include C18 (octadecylsilane), C8, or C4 chains, with C18 being the most prevalent. The retention of compounds increases with the length and surface coverage of these hydrocarbon chains—longer chains (e.g., C18) retain hydrophobic molecules more effectively than shorter ones (e.g., C8).
Mobile Phase
The mobile phase is polar, typically a mix of water and organic solvents like methanol or acetonitrile. A gradient elution is often employed, starting with a high-water content (polar) and gradually increasing the organic solvent proportion to reduce polarity. This allows less hydrophobic compounds to elute first, followed by more hydrophobic ones. Additives such as buffers adjust pH, influencing solute ionization and retention.
Retention Mechanism
Separation occurs through partitioning between the hydrophobic stationary phase and polar mobile phase:
- Hydrophobic interactions: Non-polar compounds adsorb strongly to the stationary phase primarily through Van der Waals interactions, particularly London dispersion forces, while hydrophilic compounds elute more quickly.
- Gradient elution: Increasing organic solvent concentration disrupts these interactions, selectively eluting compounds based on hydrophobicity.
- Molecular simulations reveal that C18 chains adopt flexible conformations, creating a dynamic interface where solutes partition based on their affinity for the hydrophobic chains versus the mobile phase.
Applications of Reversed-phase Chromatography
Reversed-phase chromatography is a subtype of HPLC and is widely used for separating and analyzing a variety of compounds. Reversed-phase chromatography is used across various fields:
- Pharmaceutical Analysis: Reversed-phase chromatography is used to determine the purity and potency of drug substances and products and to evaluate their stability. It's also used in the analytical separation of drugs and their metabolites.
- Environmental Testing Reversed-phase chromatography helps detect and measure pollutants like pesticides, herbicides, and volatile organic compounds.
- Biochemical Research: Reversed-phase chromatography is effective for separating proteins, peptides, and nucleic acids. It can also allow for differential separation of the proteome.
- Food and Beverage Analysis: Reversed-phase chromatography is employed to identify and quantify vitamins, sugars and carbohydrates, organic acids and amino acids.
- Metabolomics: Comprehensive analysis of metabolites and identification of biomarkers. Reversed-phase HPLC columns facilitate the thorough analysis of metabolites and the discovery of biomarkers, which are essential for diagnosing diseases and developing new drugs.
How to Choose Reversed-phase HPLC Columns?
Selecting the right Reversed-phase HPLC column is essential for achieving optimal separation, efficiency, and resolution. The key factors to consider include solid support type and column selectivity based on analyte characteristics.
Choosing the Right Solid Support
The morphology of the stationary phase significantly impacts column performance. Common solid supports include:
- Core-Shell & Organo-Silica Core-Shell
- Features a solid silica core with a porous shell.
- Provides faster chromatography and higher efficiency than fully porous particles.
- Ideal for method transfers between laboratories.
- Fully Porous Thermally Modified Silica
- High efficiency, robustness, and versatility.
- Suitable for UHPLC, HPLC, and preparative HPLC applications.
- Traditional Fully Porous Silica
- Offers excellent mechanical strength.
- Best for scaling up from analytical to preparative/process applications.
- Fully Porous-Organo Silica
- Contains organic groups, enhancing resistance to dissolution at high pH.
- Extends column longevity under extreme conditions.
Column Selectivity & Its Impact on Separation
Column selectivity is the most influential factor in achieving high resolution. It is characterized using the hydrophobic subtraction model, which considers:
- Hydrophobicity: Dominant for neutral compounds.
- Steric Influences: Affects shape selectivity and solute accessibility.
- Hydrogen Bonding
- Donating Capacity: Enhances retention of hydroxyl/amine-containing compounds.
- Accepting Capacity: Affects interaction with hydrogen bond donors.
- Cation Selectivity
- At Neutral pH: Influences ionized bases.
- At Low pH: Plays a role in retention behavior.
Matching Column Selectivity to Analyte Classes
Different analytes require specific selectivity profiles to ensure effective separation:
- Hydrocarbon/Hydrophobic Compounds
- High hydrophobicity (e.g., C18) enhances retention.
- Alternative chemistries may be needed to shorten run times without compromising separation.
- Isomers & Isobaric Compounds
- Require columns with multiple interaction mechanisms (e.g., F5 – Pentafluorophenyl).
- Compounds with Hydroxyl or Amine Groups
- Benefit from columns with high hydrogen bond capacity.
- Aromatic/Ring-Containing Compounds
- Require pi–pi interactions for enhanced retention and resolution.
- Polar Acidic Compounds, Non-Ionized Bases, Oxygen- or Halogen-Containing Compounds
- Need specific hydrogen bond donating capacities and cation selectivity for proper retention and separation.