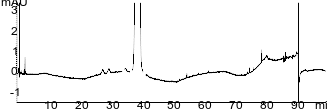
| 1Ezetimibe |
|---|
| 2o-Fluorobenzene isomer |
Applications
USP Ezetimibe Organic Impurities Standard Solution (Resolution Requirement) 0.25mg/mL on Kinetex 5u F5 150x4.6mm on Agilent 1260 Quaternary UHPLC system
27756
Separation Mode: Reversed Phase
Reversed Phase
F5
HPLC
Anticholesteremic Agents
Pharmaceutical
Pharmaceutical
Therapeutic / Clinical
USP Ezetimibe Organic Impurities Standard Solution (Resolution Requirement) 0.25mg/mL on Kinetex 5u F5 150x4.6mm on Agilent 1260 Quaternary UHPLC system
Analytes
Details
LC Conditions (App ID: 27756)
Column: |
Brand Name: Kinetex |
Part No: |
Phase Name: F5 |
System: Agilent Technologies 1260 Infinity Agilent Technologies 1260 Infinity |
Similar Applications
No Data