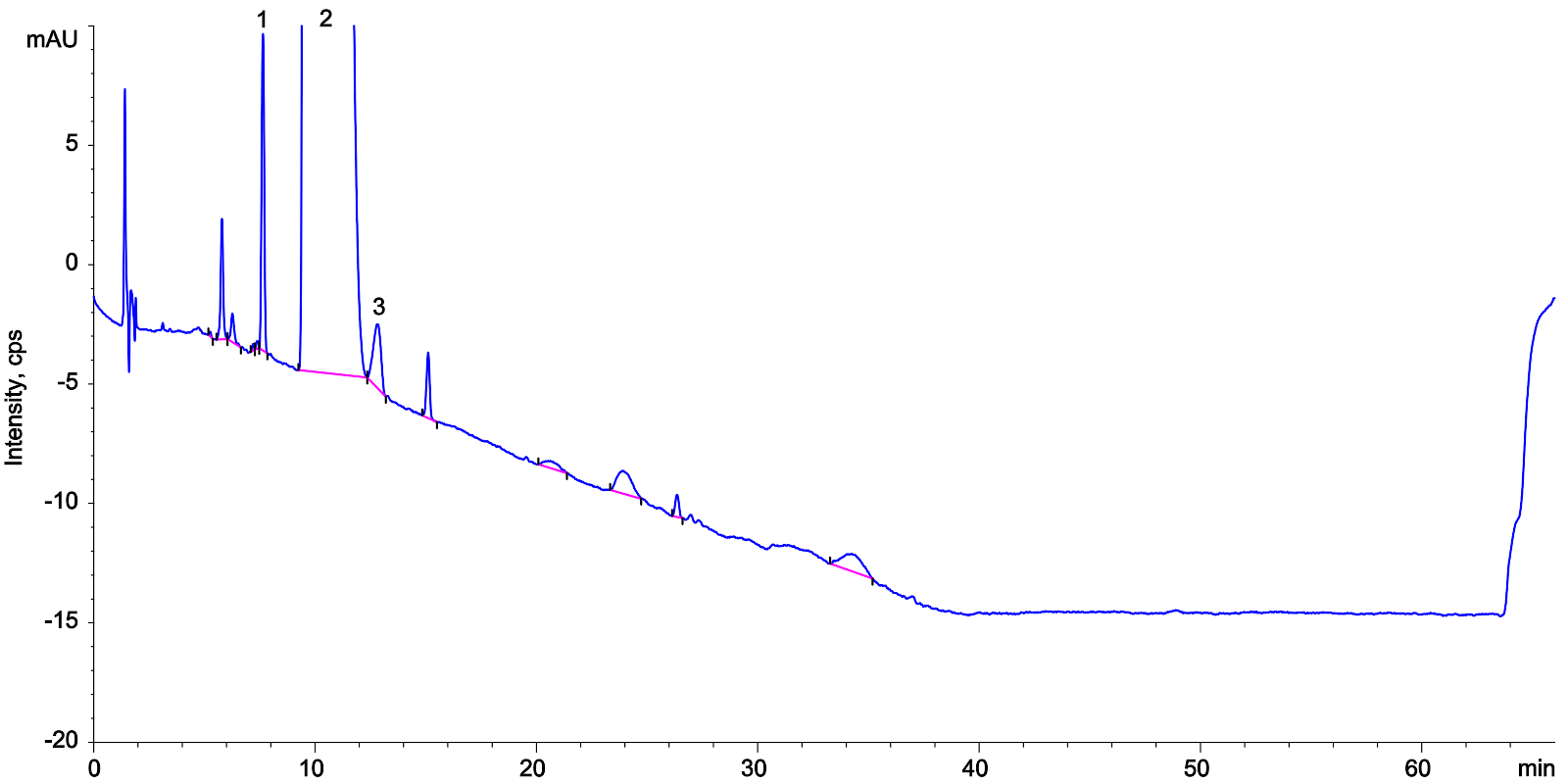
| 1Impurity A |
|---|
| 2Lisinopril |
| 3Impurity E |
Applications
Lisinopril Ph. Eur. System Suitability Reference Solution A on Kinetex 5 µm C18 250 x 4.6 mm on Agilent 1100 LC/UV
26295
Separation Mode: Reversed Phase
Reversed Phase
Ph.Eur. M.1120
C18
HPLC
Antihypertensive Agents
Pharmaceutical/Biopharmaceutical
Pharmaceutical
Therapeutic / Clinical
Lisinopril Ph. Eur. System Suitability Reference Solution A on Kinetex 5 µm C18 250 x 4.6 mm on Agilent 1100 LC/UV
Analytes
Details
Official Method: Ph.Eur. M.1120
LC Conditions (App ID: 26295)
Column: |
Brand Name: Kinetex |
Part No: |
Phase Name: C18 |
System: Agilent Technologies Agilent Technologies |
Similar Applications
search
No Data