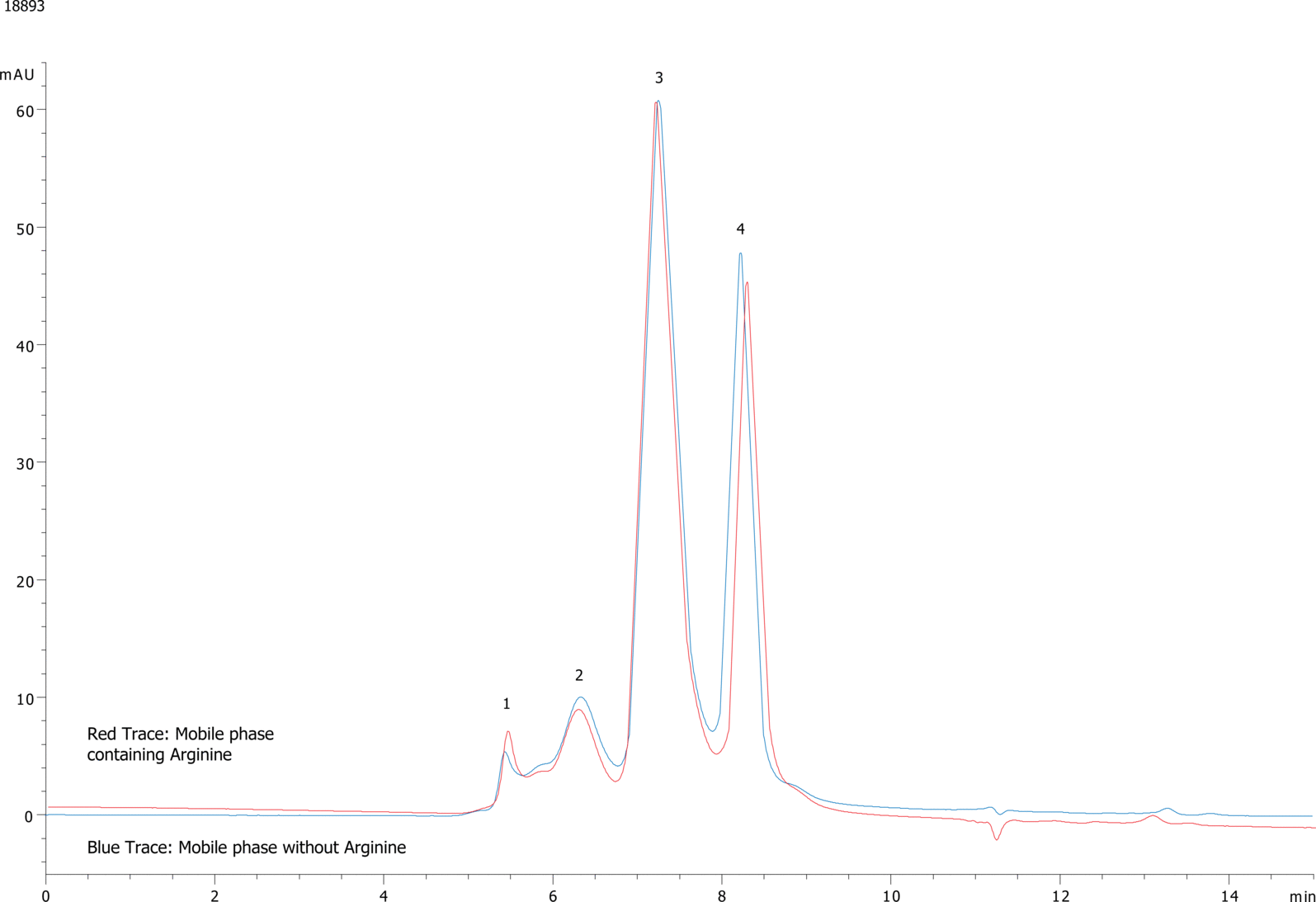
| 1aggregate |
|---|
| 2IgG dimer |
| 3IgG monomer |
| 4BSA monomer peak |
Applications
Albumin and IgG (2:3) on BioSep2000 (2)
18893
Separation Mode: Gel Filtration Chromatography (GFC)
Gel Filtration Chromatography (GFC)
SEC-s2000
HPLC
Pharmaceutical/Biopharmaceutical
Life Science
Protein
Biochemical Compound / Nutrient
Analytes
Details
LC Conditions (App ID: 18893)
Column: |
Brand Name: BioSep-SEC-S |
Part No: |
Phase Name: SEC-s2000 |
Sample Note: Application Topic: Inertness of GFC phases and accurate aggregate analysis
Protein aggregation is the post translational modification of most interest for those developing protein therapeutics. GFC Chromatography has been the "gold standard" method for quantitating aggregates in therapeutic proteins for over twenty years, however recent concerns have arisen that some GFC media may have non-specific interactions with protein aggregates leading to incorrect quantitation of aggregate in protein samples. In several papers on the subject Tsutomu Arakawa et al. (BioProcess International Nov. 2006) noted reduced recovery of aggregated Ig-G by GFC when using standard mobile phase conditions (phosphate/ salt buffers); however when arginine was added to the mobile phase recovery of aggregated protein was enhanced 3-5x over standard conditions. It is believed that arginine helps disrupt non-specific interactions between the GFC stationary phase and the protein aggregate. Thus, comparing aggregate recovery using arginine containing mobile phases to physiological mobile phases is a good inertness test of a GFC media.
In this application a mixture of Ig-G and albumin was analyzed using a BioSep 2000 using a 100 mM phosphate buffer pH 6.8 mobile phase. The analysis was repeated with mobile phase that had 200 mM or arginine added and runs were overlaid. Any significant differences in protein recovery (especially aggregated protein) between the arginine containing mobile phase and standard mobile phase would suggest non-specific interactions between the BioSep 2000 stationary phase and aggregated protein. Ig-G and albumin are especially good proteins for analyses because both proteins are well known to have interactions with other proteins and surfaces. As one can see from the overlaid chromatograms, the results are nearly identical for the Ig-G, albumin, and aggregate peaks. These results indicate that the BioSep 2000 is a very inert media unlike other commercial GFC phase on the market and demonstrate the advantages in using the BioSep media for aggregate analysis. |
Similar Applications
search
No Data